Ergin Yalcin/E+/Getty Images
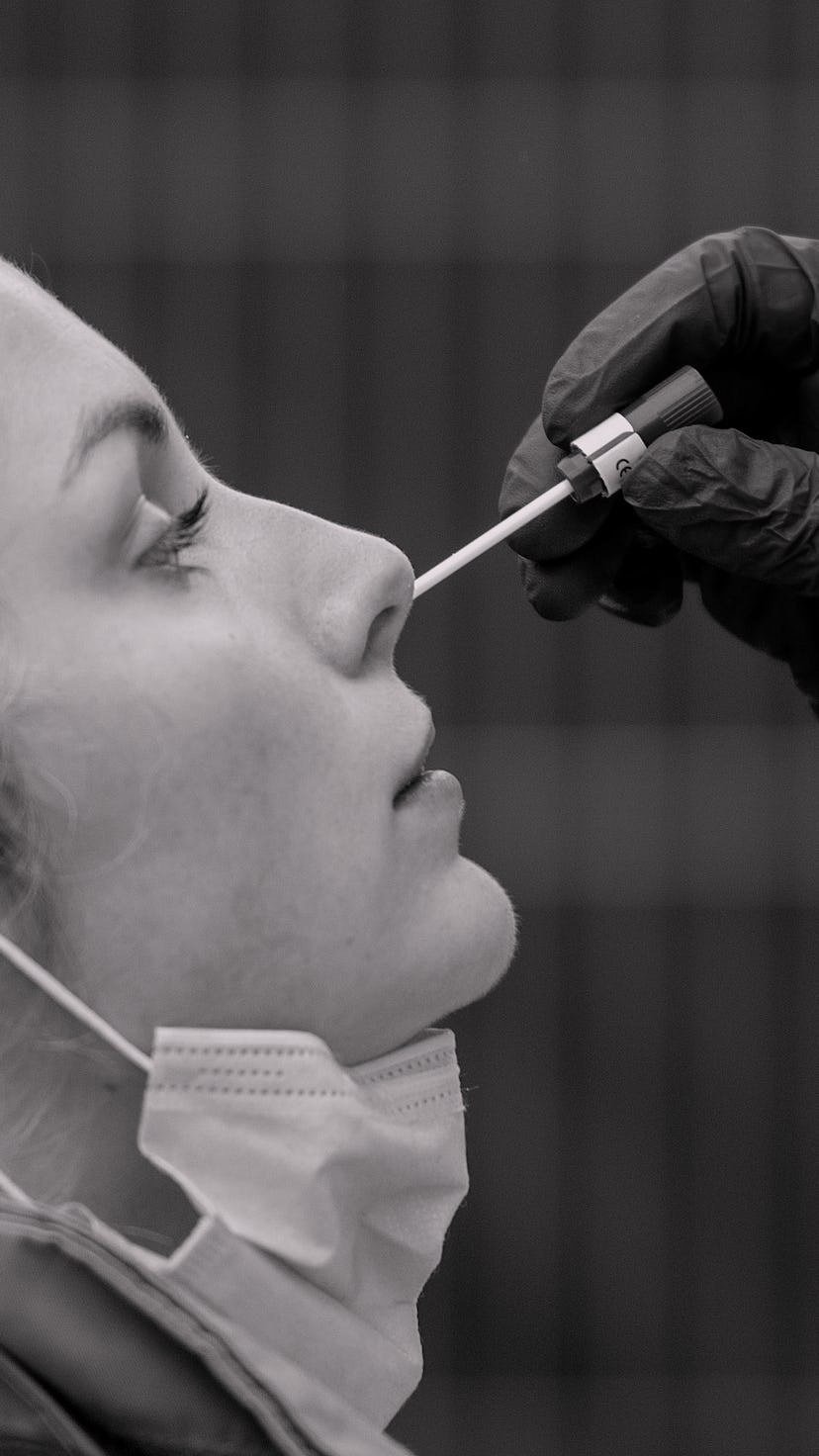
The Food & Drug Association (FDA) authorized a rapid at-home COVID-19 test on Dec. 15, reports the Washington Post. The test, made by Australian company Ellume, is the first in the U.S. that can be bought over the counter with no prescription. Even better? It'll cost around $30.
The Ellume test uses a plastic swab to deliver results within minutes. Users have to download an app to get their results. The app transmits their ZIP code to the cloud, which allows health departments to track new cases.
DjelicS/E+/Getty Images
You can't buy an Ellume test right now, though. The company will only produce 100,000 to start, CEO Sean Parsons told The Washington Post, but aims to make 20 million by mid-2021, per The Guardian. They plan to partner with pharmacy chains to supply them starting in January.
“Today’s authorization is a major milestone in diagnostic testing for COVID-19. By authorizing a test for over-the-counter use, the FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test and find out their results."
FDA Commissioner Dr. Stephen M. Hahn M.D.
Tempura/E+/Getty Images
There are other ways to test at home. The FDA approved the prescription-only at-home Lucira COVID test in November, and that comes in at around $50. Other companies offer home testing kits, but samples need to be mailed to a lab for processing.
picture alliance/picture alliance/Getty Images
The Ellume isn't as accurate as lab tests, but it’ll be convenient. "The fact that it can be used completely at home and return results quickly means that it can play an important role," FDA's Director of the Center for Devices & Radiological Health, Dr. Jeff Shuren M.D., said.
The Ellume test identified 96% of positive samples and 100% of negative samples in people with symptoms, per the FDA. In asymptomatic people, it identified 91% of positive samples and 96% of negative ones.
SUJIT JAISWAL/AFP/Getty Images
If you have no symptoms and get a positive result on an Ellume test, the FDA recommends getting another test as back-up.
FREDERIC J. BROWN/AFP/Getty Images
COVID testing in the U.S. has been beset by long wait times, unexpected costs, and shortages in 2020, but Ellume is one of several antigen tests hoping to make COVID testing easier in 2021.